Problem 1 (20 pts total)
Nitrogen is bubbled through a liquid mixture that contains initially equimolar amounts of benzene (B) and toluene (T). The system pressure is 3 atm and the temperature is 80qC. The nitrogen flow rate is 10.0 standard liters per second. The gas leaving the bubbler is saturated with B and T vapors.
- Estimate the initial rates (in mol/min) at which B and T leave the bubbler.
Nitrogen gas is bubbled through the system in which a liquid mixture of equimolar amount of benzene and toluene is mixed at
Temp= 80ºC
P = 3 atm
Temperature in Kelvin gives 80+273=353 K
The volumetric flow rate= 10L/min
From ideal gas law PV=nRT
P1V1=P2V2
Obtaining volume for benzene
Obtaining vlomue for toluene
Getting the initial rates
Benzene
Toluene
Related: Analysis of a Co-ordination Compound
- How will the mole fractions of B and T in the liquid change with time (increase or decrease or remain the same)? Explain your answer.
The mole fraction of both benzene and toluene will change with time since as the reaction continues, bonding between the atoms of benzene, toluene and nitrogen takes place. This will influence the molarity of the reactants hence triggering the change in mole fraction
- How will the mole fractions of B and T in the exiting gas change with time (increase or decrease or remain the same)? Explain your answer.
The mole of the exiting gas will decrease with time since the element in the reactant will keep bonding even though at a lower rate until most of the portion will have formed compounds that cannot leave the bubbler again.
Problem 2 (15 pts total)
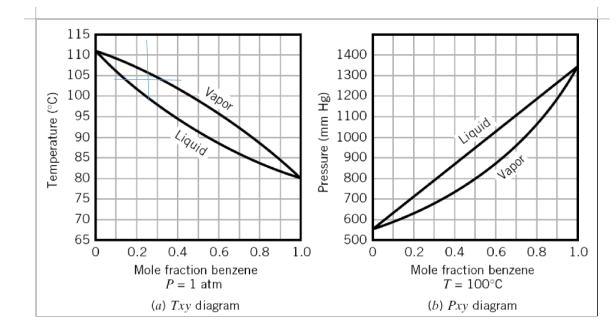
A vapor mixture containing 30 mole % of benzene (B) and 70 mole % of toluene (T) at 1 atm is cooled isobarically in a closed container from an initial temperature of 115qC. Use the Txy diagram (attached below) to answer the following questions:
- At what temperature does the first drop of condensate form? What is its composition?
Between 104º to 105º
- At one point during the process the system temperature is 100º Determine the mole fraction of B in the vapor and liquid phases and the ratio of total moles in vapor/total moles in liquid at this point.
The vapouor pressure= 0.855
The liquid pressure=0.987
Mole fractions vapour
Mole fraction for liquid
- At what temperature does the last bubble of vapor condense? What is its composition?
At the same temperature as the bubble point XB =30% XT=70%
970C <T<990C
Problem 3 (25 pts total) n-butane is converted to isobutane (see reaction below) in a continuous isomerization reactor that operates isothermally at 149qC.
The feed to the reactor contains 93 mole % of n-butane, 5 mole % of isobutane, and 2 mole % of HCl at 149qC. A 40% conversion of n-butane is achieved.
- Taking a basis of 1 mol of feed gas, calculate the moles of each component of the feed and product mixtures and the extent of reaction (in mol).
- Calculate the standard heat of the isomerization reaction (e., heat of reaction) (in kJ/mol). The taking the feed and product species at 25qC as references, prepare an inletoutlet enthalpy table and calculate and fill in the component amount (in mol) and specific enthalpies (in kJ/mol). (Siggia, 1959)
Using Hess’ Law
Ref: all components at 25ºC 1, atm
Considering that the inlet and outlet are at the same temperature
H1=H4
H2=H5
H3=H6
- Calculate the required rate of heat transfer (in kJ) to or from the reactor (state, which it is). Then determine the required heat transfer rate (in kW) for a reactor feed of 325 mol/hr.
EB on open system
Using Heat of Reaction
- Use your calculate results to determine the heat of the isomerization reaction at 149q
Problem 4 (40 pts total)
A gas mixtures containing 85 mole % of methane and the balance of oxygen is to be charged into an evacuated well-insulated 10-liter reaction CLOSED (a hint for your calculations of heat management!) vessel at 250 C and 200 kPa. An electrical coil in the reactor, which delivers heat at a rate of 100 W, will be turned on for 85 seconds and then turned off. Formaldehyde will be produced in the reaction:
CH4 + O2 HCHO + H2O
- Calculate the maximum pressure that the reactor is likely to have to withstand, assuming that there are no side reactions. If you were ordering the reactor, why would you specify an even greater pressure in your order? Give at least two reasons.
1 MOLE of gas mixture contains
0.85Moles of Methane (CH4)
0.15 Moles Oxygen (O2)
Temperature 273+25=298 K
CH4+O2=HCHO+H2O
HC(298) = -802 KJ/mol
1 MOL +1 MOL=1MOL+1MOL
0.85MOL+0.85MOL O2=0.85MOL+0.85MOL
Total moles for oxygen = 0.15+0.15= 0.3
Total moles for methane= 0.85*2= 1.7
From ideal gas equation
=11.89atm
It would have the ability to use increased severity to meet hydro treating specifications
It allows for increased operating temperature to meet products specification at lower or higher temperature without melting
- Why would heat be added to the feed mixture rather than running the reactor adiabatically?
Running the reactor adiabatically would mean that there is not heat produce hence the reaction being without heat exchange it would also require that the temperature be considered to be varying unlike as it is considered a constant.
- Suppose the reaction is run as planned, the reaction products are analyzed chromatographically, and some CO2 is detected. Where did it come from? If you had taken this CO2 into account, would your calculated pressure in part a) have been larger, or smaller, or you cannot tell without doing the detailed calculations?
During the reaction involving methane and oxygen, the formation of water and Formaldehyde would be more compared to other product. A side reaction due to the presence of head would lead to rapid bonding between carbon and oxygen since this is a reaction that takes place in the presence of the heat. The pressure would be larger since there is an additional reactant in the whole experiment.
(for problem 2)
Reference
Siggia, S. (1959). Continuous Analysis of Chemical Process Systems. University of Michigan: Wiley.