The visible light is the portion of the electromagnetic spectrum that can be seen by human eyes. There are electromagnetic radiation in the range of wavelengths in this perspective that are called the visible light. The longest lengths which the human eyes can respond on this wavelengths ranges between 400 to 700 nanometers which corresponds to the vicinity of 440-770 THz. In the spectrum all colors that can be distinguished by human eyes are not there however there are colors that are termed as the unstructured such as purple or pink while other like magenta are not present because they are made of mixtures of multiple wavelengths. There are colors with single wavelengths which are called spectral colors /pure colors. These wavelengths can pass in large number without attenuated via the earth atmosphere or through the optical windows.
Therefore the wavelength is defined as a measure of the distance between the two identical peaks called crests or between the two high points. The wavelength are used to represent the repeat of a given pattern of the energy in motion like sound and the light. They have a distinctive formations that plays a vital responsibility in differentiating different types of energies from one to another. There are specialists in various fields such as scientific and technological professionalisms in identifying the different types of energy. The space in between the repetitions in those waves shows the type of wavelengths that is found on the electromagnetic radiation spectrum. Among them were the waves in the visible light and radio waves of audio range.
Related: Analysis of a Co-ordination Compound
The term absorbance is defined as the measure of the ability of a substance or a solution that can enable it to absorb electromagnetic radiation or the light in a specific wavelength. The absorbance is presented by letter (A). The absorbance is equals to the logarithm ratio of the given radiant power of those incident radiation p, to a given power of the transmitted radiation o. In a given solution, the absorbance can be expressed as the logarithm of ratios of the radiant power of light that can be transmitted over the reference sample of the light that is transmitted through the solution.
Concentration in a chemistry is defined as the abundance of a constituent divide by the volume of the given mixture. There are various many concentrations such as volume concentration, molar concentration, mass concentration and number concentration. Concentration can be found in any kind of chemical mixture. Analytical wave length for quantitative analysis is the wavelength that corresponds to the absorption peaks.
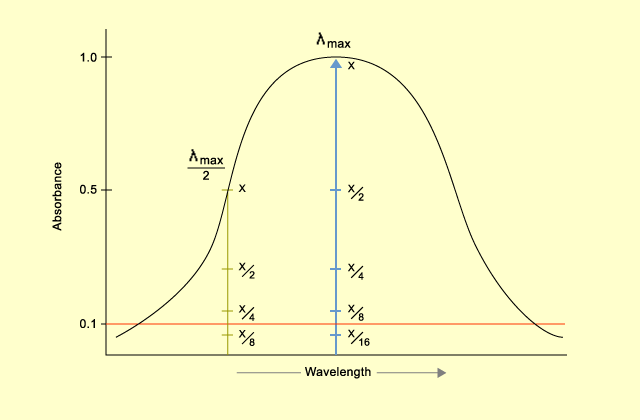
Analysis
In the experiment above the study was to determine the relationship between the color and the wave-lengths the longest and the shortest wave lengths were obtained as follows.
| able | ||
| color | Short wave length | Long wave length |
| violet | 441 | 473 |
| blue | 473 | 512 |
| green | 512 | 563 |
| yellow | 563 | 575 |
| orange | 575 | 595 |
| red | 595 | 700 |
From the experiment above, the length of the wavelength which is visible begins at 400-700. The shortest I the blue color ranging from 441-473 when the wave length is increased by 20m while for the highly visible red color ranges from 595-700 when the wave length is equally increased by 20m. The intensity of the light can be changed by turning the absorbance control by inserting chalk sample as well. As this process goes the intensity of the light changes. The amount of the energy that can be obtained in the analytical wavelength is that E=hymn use E = hν to get the energy:
E = (6.626 x 10¯34 J s) (5.4545 x 1014 s¯1)
E = 3.614 x 10¯19 J
Now, in order to get the amount of the energy that can be absorbed in one mole of cobalt (II) ions absorbed energy at the wavelength, let’s take the wavelength to be 512 nm.
Solution:
1) Convert nm to m:
512.0 nm = 512.0 x 10¯9 m = 5.120 x 10¯7 m
The atoms do bond together to form a compound because in doing that they attain lower energies than they possess individual atoms therefore the energy here is less than that of O-H bond.